We Win or You
Pay Nothing
We have staff standing by at all hours to help you.
Immediate
Medical Care
Receive the treatment you need, as soon as you need it, with usually no out-of-pocket cost to you.
24/7
Access to Us
Speak with a real human being, no matter what time it is.
Client
Satisfaction
Enjoy working alongside a firm with a sterling reputation for 1,400+ 5-Star Google Reviews.
Defective MiniMed Insulin Pumps Have Been Linked to Life-Threatening Injuries
Here’s Why & What You Need to Know
The experienced defective device lawyers at Trust Guss Injury Lawyers are currently investigating Medtronic insulin pump claims. Skilled at standing up to negligent medical device makers, we know what it takes to win these cases and help victims just like you recover from their insulin pump injuries.
To get answers about your rights and how to proceed, explore this helpful guide on Medtronic insulin pump problems, injuries, lawsuits, and more. For additional information and confidential advice, contact our defective insulin pump attorneys. We offer zero-cost, zero-obligation case evaluations for potential MiniMed insulin pump claims, and we are honored to help victims seek the justice and recoveries they deserve.
Background: What Are the Problems with Medtronic MiniMed Insulin Pumps?
MiniMed insulin pumps are designed to manage and treat type 1 diabetes in patients 16 and older. While Medtronic makes various models of insulin pumps, the 600 series was developed to automatically monitor glucose levels, delivering insulin via a “closed loop” system.
Central to the design and function of the 600 series insulin pumps are clear retainer rings that lock the insulin cartridges in place.
According to authorities, the problem with the MiniMed insulin pumps arises from:
- Damaged, broken, or missing retainer rings failing to lock insulin cartridges into place
- Too much or too little insulin delivered to patients as a result
Medtronic MiniMed Insulin Pump Injuries
Incorrect insulin doses from defective MiniMed insulin pumps can result in some severe injuries, including:
- Hypoglycemia: Describing dangerously low blood sugar (glucose) levels, hypoglycemia can be characterized by seizures, arrhythmia, confusion, dizziness, and loss of consciousness. This complication can occur when damaged retainer rings detach from MiniMed insulin pumps, delivering large amounts of insulin to patients in a short period of time.
- Hyperglycemia: Referring to excessively high blood glucose levels, hyperglycemia is generally marked by nausea, vomiting, fatigue, extreme thirst, and loss of consciousness. This severe insulin pump complication, which may lead to diabetic ketoacidosis, can arise when the retainer ring fails to lock the insulin cartridge in place, creating a gap between the pump and the cartridge that prevents the pump from delivering the proper doses of insulin to patients.
Hypoglycemia and hyperglycemia can both progress to deadly stages without intervention and the proper medical treatments.
FDA Announces Class I Recall for Faulty Medtronic MiniMed Insulin Pumps
On November 21, 2019, Medtronic, Inc. recalled roughly 463,460 MiniMed insulin pumps in the U.S. With this recall, authorities also announced that they had received upwards of 26,000 reports of malfunctioning MiniMed insulin pumps, including thousands of patient injuries.
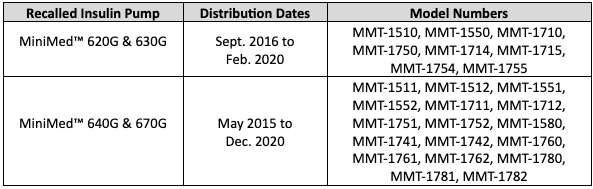
The models and distribution dates for the affected devices are featured in the table below.
With life-threatening and fatal injuries linked to MiniMed insulin pumps, the U.S. Food and Drug Administration (FDA) elevated this response to a Class I recall, the most serious type of recall for medical devices and drugs. At the time, patients were advised to:
- Immediately examine the retainer ring on their insulin pump.
- Stop using their insulin pump if it has a damaged, broken, or missing retainer ring.
- Manually administer insulin until they get a new insulin pump.
On October 5, 2021, Medtronic, Inc., updated its recall for the MiniMed insulin pumps, notifying patients that they should replace any insulin pump that has a clear retainer ring, regardless of whether that retainer ring was damaged. The company explained that it would replace the older devices with newer ones that feature black retainer rings, “regardless of the warranty status of the pump.” Patients can request replacement devices here or by calling 1-877-585-0166.

Other Medtronic MiniMed Recalls
Tragically, the 600 series MiniMed insulin pumps are not the only devices that Medtronic has had to recall due to issues that have allegedly harmed patients. In fact, over the years, Medtronic has been at the forefront of several recalls, including (but not limited to) recalls in:
- August 2018: This Medtronic recall, impacting more than 31,000 devices, was issued due to cybersecurity risks associated with the remote controllers for the MiniMed 508 insulin pump. Like the recall for the 600 series, the 2018 recall was also classified as a Class I recall.
- September 2017: Pertaining to a MiniMed infusion set, this recall was issued to address potential interference with the fill-tubing (or “priming”) process.
- September 2015: Focused on the MiniMed 640G insulin pump, this recall was rolled out due to pump drive motor malfunctions.
Remarkably, insulin pumps are one of the riskiest medical devices on the market when looking at patient injury reports. In fact, one investigation found that:
- While metal hip replacements are associated with the most patient reports of device-related injuries, insulin pumps rank second in this category.
- Insulin pump problems can be easier to overlook or attribute to user (patient) error rather than underlying defects associated with the device itself.

Medtronic MiniMed Insulin Pump Lawsuits
Thousands of MiniMed insulin pump lawsuits have already been filed against Medtronic, Inc., generally alleging that:
- Defects with the MiniMed 600 series insulin pumps were directly responsible for harming patients.
- Medtronic knew about or should have known about the problems with their 600 series insulin pumps.
- Medtronic failed to take sufficient action to warn the public about the problems with their insulin pumps and/or promptly recall them upon receiving reports of patient injuries.
As of December 2022, the pending MiniMed lawsuits against Medtronic have not been consolidated into multidistrict litigation (MDL). Nevertheless, this action may be ordered if additional claims are filed in the coming months.
Regardless of whether an MDL is formed, these insulin pump lawsuits filed by patients are just one set of legal actions that Medtronic is now fighting. Additionally, the company is also facing off with investors who have accused Medtronic of lying about the insulin pumps’ safety issues in an effort to inflate the value of the
Do I Have a Medtronic MiniMed Insulin Pump Claim?
If you or someone you love has experienced serious injuries that have been linked to a Medtronic MiniMed insulin pump:
- You may have the right to sue: Lawsuits have already been filed by several patients who have suffered hypoglycemia, hyperglycemia, and related complications after using the MiniMed insulin pump. Patients in these cases are seeking damages for their medical bills, lost earnings, pain, suffering, and more.
- You don’t have forever to file an insulin pump claim: There are strict deadlines for filing Medtronic insulin pump lawsuits, and missing the deadline can mean forfeiting the right to seek compensation. That’s why it’s so crucial to consult an attorney ASAP and find out more about a potential insulin pump case.
Talk to a Defective Device Attorney Now
A trusted mass tort lawyer at Trust Guss Injury Lawyers is ready to explain your rights, share important advice, and help you 24/7. CLICK HERE for your free insulin pump case evaluation now.
Get a Free Consultation
2 minute response
24 hours a day, 7 Days a Week
Dedicated Trust Guss Intake Team
"*" indicates required fields

 2 minute response
2 minute response 24 hours a day, 7 Days a Week
24 hours a day, 7 Days a Week Dedicated
Dedicated